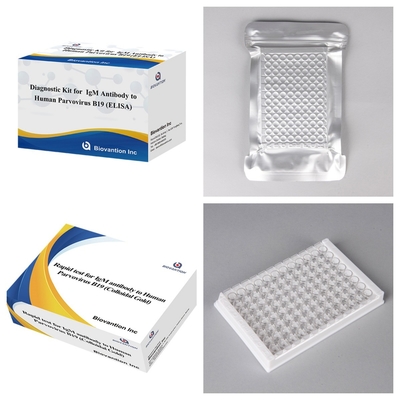
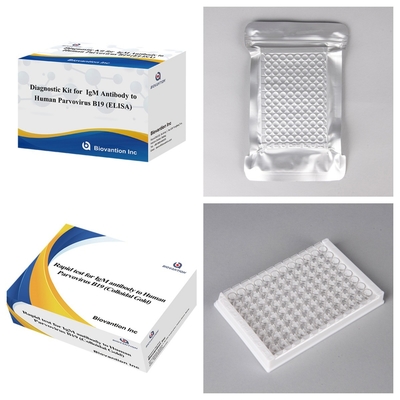
Lasciate un messaggio
Ti richiameremo presto!
 Il tuo messaggio deve contenere da 20 a 3000 caratteri!
Il tuo messaggio deve contenere da 20 a 3000 caratteri!
 Si prega di controllare la tua email!
Si prega di controllare la tua email!
Invia
Ulteriori informazioni facilitano una migliore comunicazione.
Sig.
- Sig.
- Signora
ok
Inviato con successo!
Ti richiameremo presto!
ok
Lasciate un messaggio
Ti richiameremo presto!
 Il tuo messaggio deve contenere da 20 a 3000 caratteri!
Il tuo messaggio deve contenere da 20 a 3000 caratteri!
 Si prega di controllare la tua email!
Si prega di controllare la tua email!
Invia